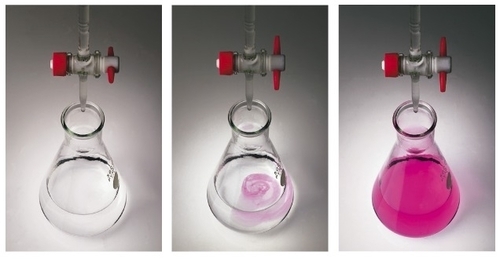
The free acid and anhydride contents are determined according to ISO 2114 by titration of the resin dissolved in appropriate solvent with standard KOH solution in methanol, in the presence of the phenolphthalein indicator. The standard provides two methods:
i) Method A with a toluene-ethanol solvent mixture, recommended for binders, paints and varnishes (where small amounts of free anhydrides are generally present). Method A determines the partial acid value (PAV) corresponding to the neutralization of all terminal carboxylic groups and free acids plus half the free anhydrides contained in a resin.
ii) Method B with Pyridine-MEK-water solvent mixture, suitable for unsaturated polyester resins in which there are also considerable amounts of free anhydrides. Method B determines the total acid value (TAV) corresponding to the neutralization of all terminal carboxylic groups, acids and free anhydrides contained in a resin.
With the TAV value, it is possible to calculate the degree of conversion p, known as the TAV0 of the reference solution of the monomer mixture.
Chemical analyses
Determination of the acid number for polyester resins (code O.1)
Determination of the epoxy equivalent (code O.2)

The UNI EN ISO 3001 standard specifies a method for determining the epoxy equivalent applicable to all epoxy compositions, including epoxy amines. It is defined as:
i) epoxy equivalent (EE): resin mass, in grams, containing one mole of the epoxy group
ii) epoxy index (EI): number of moles of epoxy groups contained in 1 kg of resin.
Epoxy groups in a portion of the test, dissolved in anhydrous acetic acid, are reacted with hydrogen bromide created in situ by a perchloric acid solution of tetraethylammonium bromide. The end of the reaction is determined using the violet crystal as the indicator.
swelling measurements of crosslinked elastomers (code O.3)
The evaluation of the crosslinking degree in elastomers can be determined by the action of liquids by measuring the properties of the elastomers before and after immersion in specific liquids. Liquids under consideration are organic solvents, chemical reagents as well as reference liquid liquids.
The action of a liquid on the elastomer may generally result in:
a) absorption of liquid
b) extraction of soluble components
c) chemical reaction
The amount of absorption (a) is generally greater than that of the extraction (b) and this is called as "swelled" volume increase. The standard defines the methods to determine:
i) weight, volume and size variation
(ii) extracted components
iii) change of mechanical properties after swelling.